"The novelty of our coupling reaction is the ability to regenerate the two initial partners at will with no alternation," says Dr. Eric Anslyn, principle investigator in the study. "This allows a protein to be decorated at surface exposed lysines with any thiol, all of which can be removed at a later time."
Click chemistry describes a type of chemical reaction that is often used to build libraries of compounds. It employs simple reactants that do not require pre-treatment, such as protection, and it usually involves a one-pot reaction in which the reactants are combined using mild conditions and it requires little-to-no purification. Click chemistry was inspired by biomolecules that are built modularly, such as peptides that are built by combining amino acids via a condensation reaction.
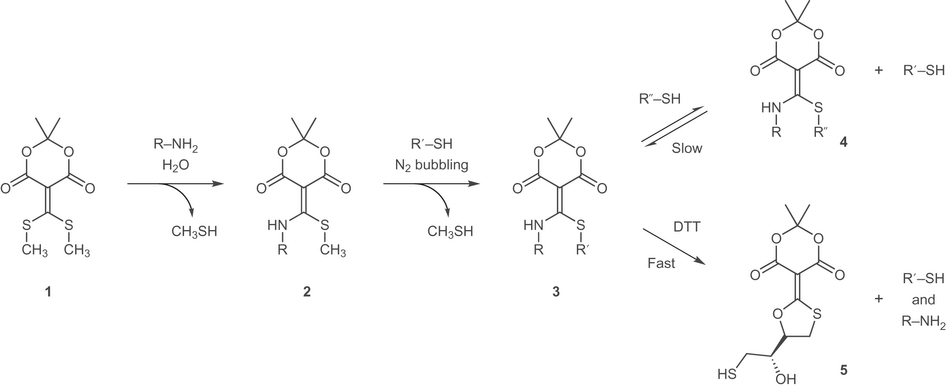
In the current study, Diehl, et al. have built upon the group's prior research involving a Meldrum's acid derivative (1) which serves as an electrophile in a subsequent amine and thiol coupling reaction. The carbon situated between the two carbonyls in the six-membered ring is an alkene with two thiomethyl substituents. These thiomethyls are sufficiently electrophilic that one of the thiols is replaced by an alkyl amine when reacted in aqueous conditions. In neutral aqueous solvent the amine is not sufficiently nucleophilic to add across the second thiomethyl site, but an alkyl or aromatic thiol is, leading to the ability to do subsequent amine and thiol coupling reactions.
Additional studies to determine the mechanism and versatility of the Meldrum's acid derivative showed that the sequential coupling reactions are pH and solvent-controlled. At neutral pH in aqueous solvent, the amine adds to only one electrophilic site. Increasing the pH affects the rate of the thiol coupling reaction and can prevent the addition of the thiol across the second site. The thiol coupling reaction is also dependent on several other factors such as the pKa of the alkylthiol and maintaining a sufficiently de-oxygenated environment to prevent disulfide formation.
To decouple both the amine and the thiol (i.e., the declick reaction), Diehl, et al. used dithiothreitol (DTT), a relatively inexpensive reducing agent. While other 1,2- and 1,3-dinucleophiles were tested, the declick reaction with DTT was faster, particularly when the reaction was heated. There are compelling reasons to believe that the reaction proceeds via a mechanism that is analogous to peptide hydrolysis reactions. The authors note that cysteine is a viable alternative for the declick reaction if the conditions requires a gentler reagent.
Finally, the authors tested their Meldrum's acid derivative with several systems to evaluate the potential applications of a facile decoupling reaction in which the thiol and amine reactants are retrieved. This process has applications for dynamic combinatorial chemistry and polymer chemistry. But, perhaps one of its most compelling applications is that of a peptide or protein tag.
A good label or tag would require a reversible reaction that operates under physiological conditions, and upon removal, the original starting material could be retrieved. These are properties of the click and declick reactions reported here. One test to see how this reaction could work for protein tagging involved reacting the electrophilic coupling reagent with lysine residues in equine myoglobin followed by a thiol exchange. This resulted in a PEGylated protein at specific sites that was reproducible. The tag was readily removed from the myoglobin using DTT.
This research demonstrates a unique reversible coupling reaction using a reagent with two equivalently electrophilic reaction sites that change upon addition to one site. This reaction is versatile for several types of amines and thiols, including ones that are biologically relevant.
Dr. Anslyn says that in regards to future applications of this work, "We are currently creating polymeric materials between amines and thiols that can be patterned at surfaces, followed by removal of the Meldrum's acid linking group, thereby creating molecular sized features in the materials."
More information: Katharine L. Diehl et al. Click and chemically triggered declick reactions through reversible amine and thiol coupling via a conjugate acceptor, Nature Chemistry (2016). DOI: 10.1038/nchem.2601
Abstract
The coupling and decoupling of molecular units is a fundamental undertaking of organic chemistry. Herein we report the use of a very simple conjugate acceptor, derived from Meldrum's acid, for the sequential 'clicking' together of an amine and a thiol in aqueous conditions at neutral pH. Subsequently, this linkage can be 'declicked' by a chemical trigger to release the original amine and thiol undisturbed. The reactivity differs from that of other crosslinking agents because the selectivity for sequential functionalization derives from an altering of the electrophilicity of the conjugate acceptor on the addition of the amine. We describe the use of the procedure to modify proteins, create multicomponent libraries and synthesize oligomers, all of which can be declicked to their starting components in a controlled fashion when desired. Owing to the mild reaction conditions and ease of use in a variety of applications, the method is predicted to have wide utility.