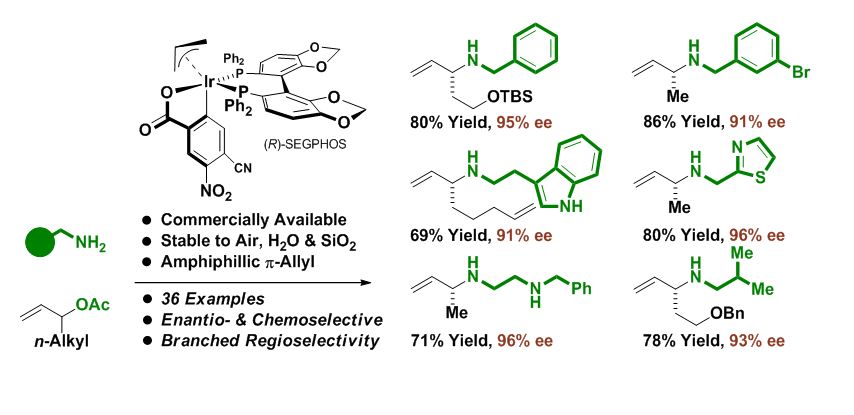
Professor Michael J. Krische and collaborators at Genentech have developed a highly enantioselective method for C-N bond formation. Krische’s signature π-allyliridium C,O-benzoates, which are commercially available and known to catalyze nucleophilic allyl acetate-mediated carbonyl allylation, are now shown to catalyze asymmetric electrophilic aminations of branched allylic acetates, representing the first examples of amphiphilic reactivity in the context of metal catalysis. This work appears as a communication in the Journal of the American Chemical Society.